CAR-T cells have potent effects to clear residual tumor
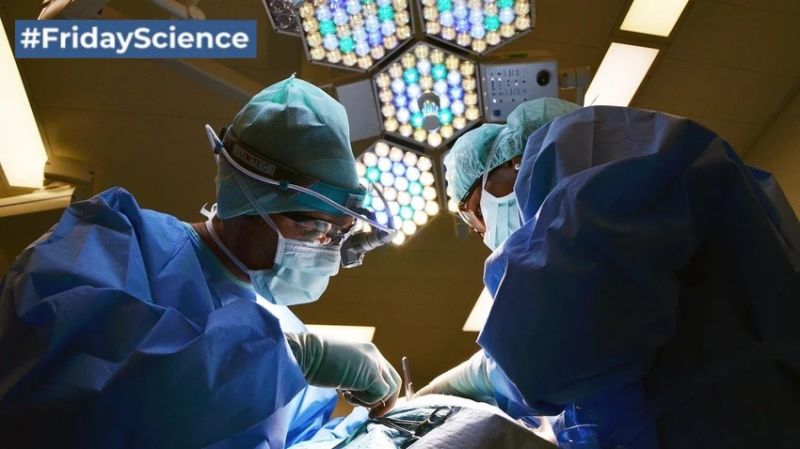
FridayScience today features a promising oncology therapy: “CAR-T cells have potent effects to clear residual tumor after incomplete resection in a variety of preclinical models” Researchers from University of Pennsylvania described how they added chimeric antigen receptor T cells to a gel (fibrin sealant) designed to prevent bleeding after surgery. After adding the gel to […]
We reduce the complexities of your drug development journey

What fuels our ambition to deliver frictionless experiences for our partners and help them succeed with faster medicine development? Designing solutions that take their molecule from the complex phase 1 trials through a regulatory process under one dedicated study manager. Thanks to years of clinical trials experience and excellent in-house team of experts we can […]
Precisely-tailored program to support trial execution

In today’s R&D world, there is no success without in-depth therapeutic knowledge to support trial execution. Our interdisciplinary team at Biokinetica has broad therapeutic expertise. Combining that with our world-renowned expert network, allows us provide precisely-tailored program to help you succeed in drug development.
FDA no longer needs to require animal tests

Today in FridayScience: In order for a drug to be approved in the US, FDA typically requires toxicity tests on animal species. The agency also supports alternative methods that are backed by science and provide the necessary data showing whether products are safe and effective. Some suggest that in clearing drugs for human trials the […]
The early clinical development of new analgesics

We invite you to join the last webinar this year organised by EUFEMED on early development of new analgesics. Sign up directly through the event. This is the fourth and last 2022 EUFEMED webinar. If you have already registered for all four webinars, you do not have to register seperately for this one. Online Christmas […]
Why Phase I CT volunteers receive financial compensation?

Have you ever wondered why Phase I Clinical Trial volunteers receive financial compensation? Healthy volunteers who devote themselves to science have many burdens to overcome, such as hidden financial costs like travel, food, or caregivers. Partly because of these burdens, patient recruitment and retention are two of the most #challenging aspects of running clinical trials, […]
Large language models help decipher clinical notes

Today in FridayScience: Massachusetts Institute of Technology researchers used a powerful deep-learning model to extract important data from electronic health records that could assist with PersonalizedMedicine. The enormous amount of information in these now-digital records could be used to answer very specific questions beyond the scope of clinical trials: What’s the right dose of this […]
We are hiring: Pharmacist

This is an exciting opportunity to join Biokinetica and play a part in our continued growth. Please take a look and share to anyone who you think would be a good fit for this role at careers page.
Why big pharma has abandoned antibiotics
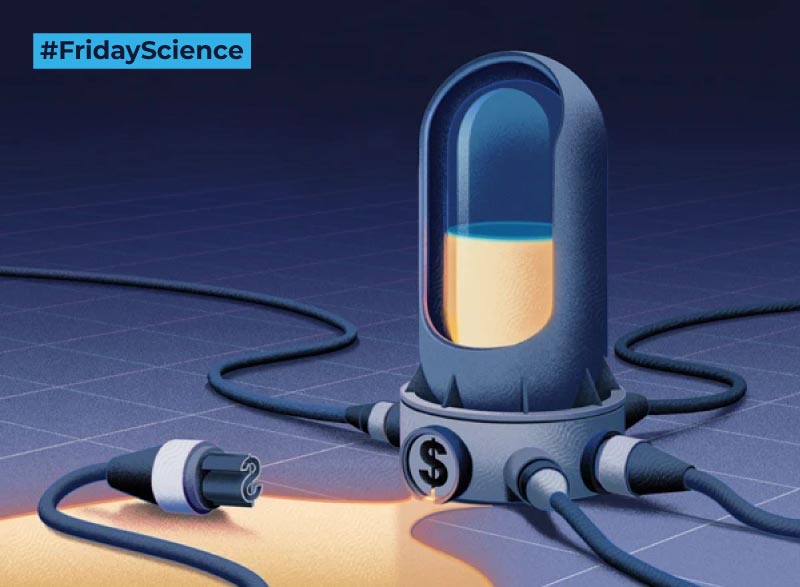
Today in FridayScience: Antibiotic resistance is the ‘silent epidemic’ due to many diseases progressively developing resistance to the antibiotics prescribed to treat it. Every year, #AMR infections lead to 700,000 deaths worldwide with the United Nations alarming they can reach 10 million a year by 2050 if no action is taken. The scarcity of new […]
Biokinetica’s Phase 1 Unit has been inspected twice by FDA!

We are pleased to inform you that Biokinetica’s Phase 1 Unit has recently undergone a FDA inspection on two of our biosimilar studies. The results demonstrated GCP compliance, and our commitment towards the highest operative quality standards of our teams. We continually strive for excellence and efficiency to deliver quality services that are tailored to […]