BK offers conduct of the clinical research services via state-of-the-art technology solutions selected carefully to ensure the highest quality of the clinical processes combined with relevant and adjusted level of customization. Clinical systems are licensed from leading technology providers – BK qualified vendors and solution partners, ensuring best in class technology solutions for our clients.
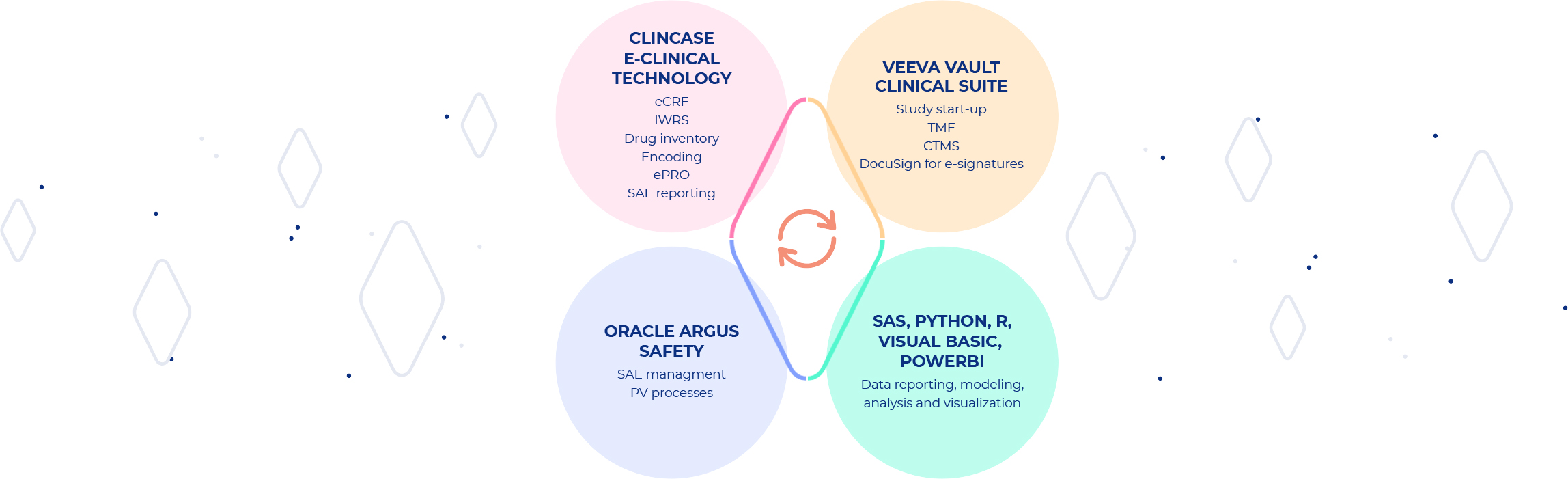

VEEVA VAULT CLINICAL SUITE combines Study-start- up, eTMF and CTMS (Clinical Trial Management System) built on a single Veeva Vault Platform that meets all rigorous usability, scalability, performance, validation and security requirements of the life sciences industry.
CLINCASE E-CLINICAL TECHNOLOGY delivers end-to-end EDC and clinical data management (CDM) system on a unified platform ensuring highly customizable and simple-to-use, straightforward functionalities.


ORACLE ARGUS SAFETY is a comprehensive adverse event (AE) management platform, it is the market-leading solution for processing, analyzing, and reporting adverse event cases originating in pre- and post-market drugs, biologics, vaccines, devices, and combination products.
Effective study implementation through a unique object-orientated approach and metadata structure. Full control over data import and export formats, simplified process of exporting data for statistical analysis.
DOCUSIGN delivers the most trusted and widely used e-signature solution to accelerate finalization/approval of documents with status/progress tracking in real- time. DocuSign eSignature complies with the U.S. ESIGN Act, HIPAA and UETA, EU eIDAS regulation as well as the e-signature practices set forth in EMA/ICH guidelines and FDA 21 CFR Part 11 with tailored functionality and packaged service offerings.