Studies with healthy volunteers and patients requiring in-house stay are performed in our modern, hospital-based, clinical research site located in the south of Warsaw.
The clinical unit is located on the campus of a hospital about 30-minutes’ drive from the center of Warsaw and Warsaw airport.

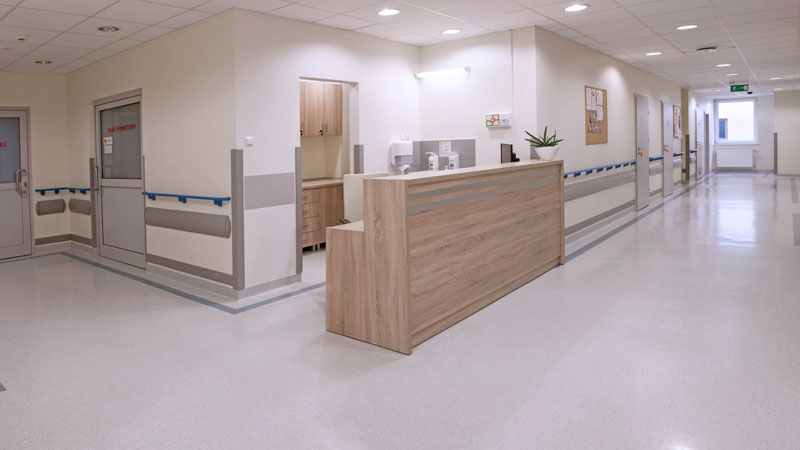
Our Clinical Site is located on the campus of a Cardiology Centre is a modern clinical ward with 2 – 4 bedrooms ideally equipped for First-in-Man studies, studies with intensive cardiac monitoring e.g. in patients with cardiovascular disorders.
The site is run by an experienced clinical team of physicians, nurses and technicians with long term experience in clinical pharmacology.


Biokinetica drives an outpatient clinic located in the center of Warsaw where 16 physicians (specialized in different medical areas) perform ambulatory examinations. The outpatient clinic has a capacity of up to 60 visits per day and is dedicated for:
Drug preparation and storage, as well as repackaging, labelling, and blinding of the IMP take place in Biokinetica’s pharmacy (area & equipment located within the site). All procedures within the pharmacy are covered by our qualified pharmacy team (three certified pharmacists).
Risk Identification and Mitigation Plan
From the beginning of the pandemic Biokinetica identified and assessed the risk, as well as developed and implemented Covid-19 Risk Mitigation Plan, which is constantly updated.
The aim of these risk mitigation procedures is to allow uninterrupted and safe conduct of clinical trials with large cohorts of healthy volunteers (this procedure applies to patient studies as well). Additionally, these procedures (including testing for SARS-Cov-2 and quarantine) are required by the Bioethics Committee, so most sponsors decide to include Covid-19 risk mitigation tests and procedures in the study protocol. This is extremely important in trials with biological compounds.

Taking into account the current epidemic situation in Poland (the third wave – currently about 15k cases daily), the implemented procedures guarantee safe conduct of the studies according to the planned timelines and goals. We’ve completed several large biosimilar projects during the pandemic, all within set timelines for recruitment/ enrolment and DB close.
Additionally, Biokinetica has created and maintains dedicated premises for the Covid-19 procedures: isolation rooms with beds, a swab room and a PCR laboratory (quick RT Real-Time PCR method used on an ongoing basis to test personnel and participants with symptoms on an ad-hoc basis). Routinely, tests for SARS-Cov-2 in the full PCR method are performed in a contracted laboratory (turn around time is up to 48 h).